Balance the Following Reactions Which Occur in a Basic Solution
What are the coefficients in front of Cl2 and OH in the balanced reaction. Please explain your answers step by step and legibly.
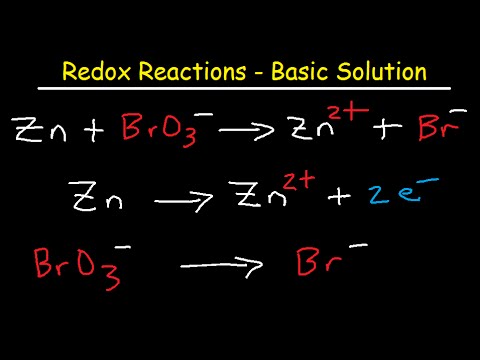
How To Balance Redox Equations In Basic Solution Youtube
A Br03 aq Br- aq Br2 1 This question hasnt been solved yet.
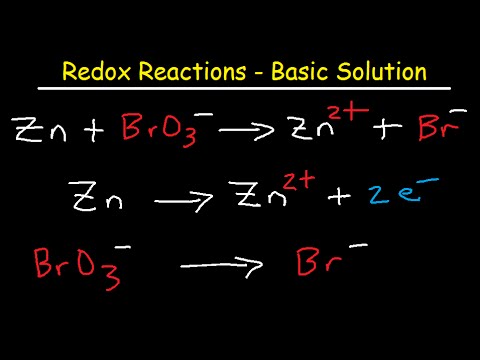
. Balance the following reactions which occur in a basic solution. Balance the following oxidation-reduction equations. Balance the following oxidation-reduction equations.
The coefficient has been placed in the chemical reaction to balance the number of atoms on the product and the reactant side. The reactions occur in acidic or basic aqueous solution as indicated. Complete and balance the following disproportionation.
The following reaction occurs in basic solution. Balance the following redox reaction if it occurs in basic solution What ae the from CHEM 1032 at Temple University. Crs Cl2gâ Cr3 aq Cl-aq.
10 pts Balance the following reactions which occur in a basic solution and show your work. This problem has been solved. The reactions occur in acidic or basic aqueous solution as indicated.
Separate the equation into two half-reactions. Who are the experts. CuS NO3 Cu2 NO S8 acidic.
The balanced reaction needs to be modified to remove the H ions and include OH - ions. 6 -OH Br - aq ---- BrO3- aq 6 e-. CN MnO4 CNO MnO2 basic c.
Balance the following oxidationreduction reactions that occur in basic solution. Br- aq ---- BrO3- 6 e- Since the reaction occurs in basic solution to balance the charge we add hydroxide ions to the left side. Br2 SO2 Br SO42 acidic e.
Given the reaction as BrO3- aq Br- aq. The number of atoms of Br on the product side has been 2 and. MnO₄ CN MnO₂ CNO Solution.
Reduction Reactions That Occur In Basic Solution stores search commencement by shop shelf by shelf it is really problematic. What are the coefficients in front of Cr and Cl 2 in the balanced reaction. Cl2 g ClO3 aq Cl aq - ScieMce.
If the reaction takes place in a basic medium the reaction can be given as. None of the above. Cr s Cl 2 g Cr 3 aq Cl - aq A Cr 2 Cl 2 3 B Cr 2 Cl 2 6 C Cr 1 Cl 2 1 D Cr 2 Cl 2 1 E none of these.
We review their content and use your feedback to keep the quality high. Balance the following reactions which occur in a basic soultion. Balance the following redox reaction if it occurs in basic solution.
A MnO4 aq Br- aq MnO2 s Br03 aq b 103 aq aq 12 s. Experts are tested by Chegg as specialists in their subject area. Balance the following redox reaction if it occurs in basic solution.
In basic solutions there is an excess of OH - ions. Balance all atoms other than H and O. Ag Cu Ag Cu2 When the equation is balanced the sum of the coefficients is.
Up to 256 cash back A disproportionation reaction is an oxidationreduction reaction in which the same substance is oxidized and reduced. When the following redox equation is balanced with smallest whole number coefficients the coefficient for Sn OH3- will be Bi OH3 s Sn OH3 aq Sn OH62- aq Bi s basic solution asked Sep 3 2019 in Chemistry by WhiteSea. The coefficient for Br and OH has been 3 and 6 respectivelyThus option C is correct.
Recombine the half-reactions to form the complete redox reaction. CH3NH2aqH2OlCH3NH3aqOHaq The pH of 265M CH3NH2aq is 1254. MathrmMnO_4-mathrmI- longrightarrow mathrmMnO_2mathrmIO_3- basic b.
Complete and balance the following redox equation. Cl2 2 OH 5. MnO₄ MnO₂ 2H₂O CN H₂O CNO.
ClOaq CrOH3s Claq CrO42aq. This will balance the reaction in an acidic solution where there is an excess of H ions. Balance the following reactions which occur in a basic solution.
Balance the following redox reaction if it occurs in basic solution. BrO-3 aqBr- aq - Br2 l Show transcribed image text. Determine the value.
Cl2 3 OH 6. Cl2 3 OH 3. The following reaction occurs in basic solution.
Cl2 g ClO3 aq Cl aq asked Aug 10 2019 in Chemistry by porter404116. MnO₄ MnO₂ CN CNO Step 2. A Br03 aq Br- aq Br2 1 Question.
Asked Jun 19 2017 in Chemistry by Harribo_Lover. MathrmCr_2 mathrmO_72-mathrmCl- longrightarrow mathrmCr3mathrmCl_2 acidic. A Ni aqNi 2 aq Nis acidic solution b MnO 4 2-aqMnO 4-aq MnO 2 s acidic solution c H 2 SO 3 aqSs HSO 4-aq acidic.
See the answer See the answer done loading. CrOH4 OCl CrO42 Cl basic d. Balance O by adding H₂O to the deficient side.
Balance the following equation in basic solution. The following chemical equation represents the reaction that occurs when methylamine dissolves in water to form a basic solution. Solution for Write a balanced chemical equation for the following reaction in a basic solution.
Balance the equation and identify the oxidizing agent. What are the coefficients in front of Cr and Cl2 in the balanced reaction. Show transcribed image text.
Balance the following reactions which occur in a basic solution. 2 aq NH 3 aq aq Zn OH 4. Cl2 1 OH 2.
It will agreed ease you to look guide balance the following oxidation reduction reactions that occur in basic solution as you such as. C r s C r O 4 2 a q C r O H 3 s mathrmCrsmathrmCrO_42-a q rightarrow mathrmCrmathrmOH_3s Cr s CrO 4 2 a q Cr OH 3 s. Thank you unquestionably much for downloading balance the following oxidation reduction reactions that occur in basic solutionMost likely you have knowledge that people have see numerous time for their favorite books once this balance the following oxidation reduction reactions that occur in basic solution but stop taking place in harmful.
Cr2O72 H2O2 Cr3 O2 acidic b. This is why we provide the ebook compilations in this website.

Balancing A Redox Equation In Basic Solution Worked Example Video Khan Academy
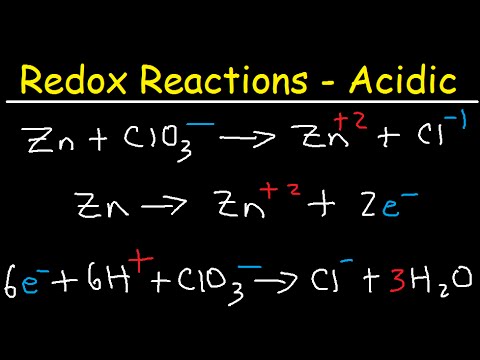
How To Balance Redox Equations In Acidic Solution Youtube
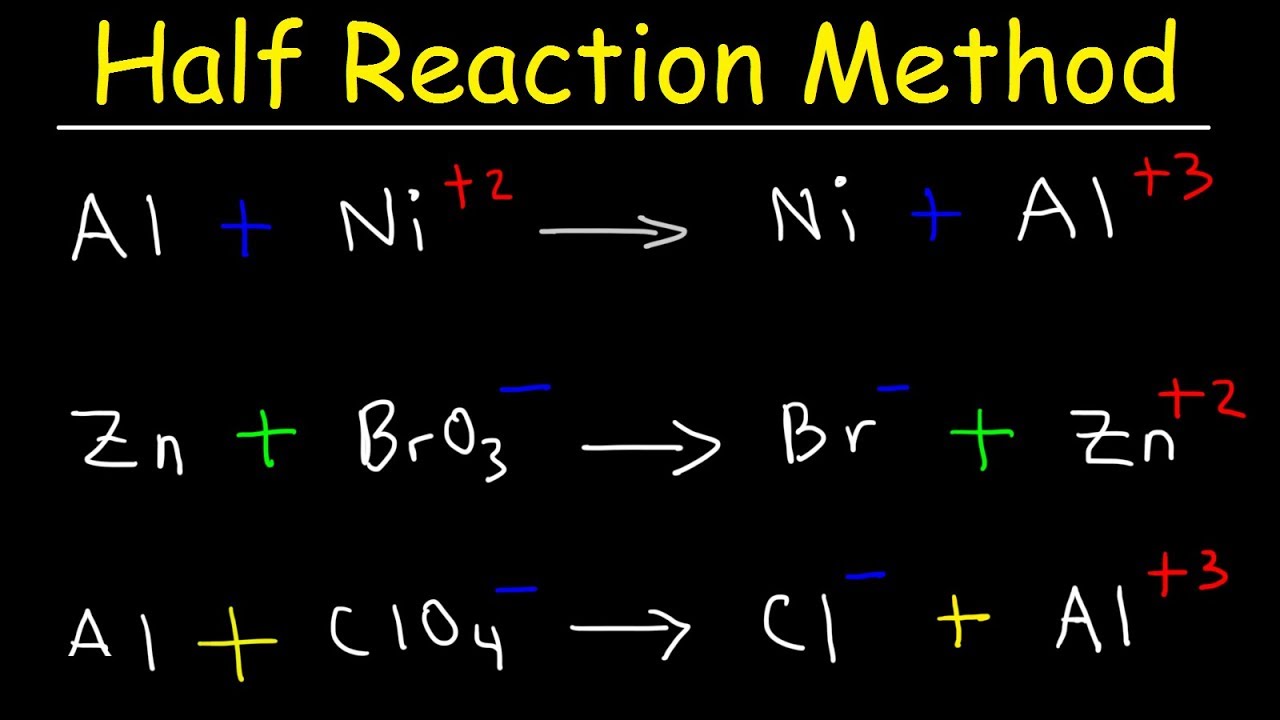
Half Reaction Method Balancing Redox Reactions In Basic Acidic Solution Chemistry Youtube
No comments for "Balance the Following Reactions Which Occur in a Basic Solution"
Post a Comment